Polyglycolic Acid Surgical Sutures for Wound Closure
2019-01-30 05:30:52
Model NO.: USP 4/0
Logo Printing: Wego or OEM
Box: 12 Pieces/Box
Carton: 100 Boxes/Carton
Trademark: Wegosuture or OEM brand
Transport Package: Aluminum Pack and Blister Pouch with Sterilize Ind
Specification: Ep, Usp
Origin: China
HS Code: 9018322000
Model NO.: USP 4/0
Logo Printing: Wego or OEM
Box: 12 Pieces/Box
Carton: 100 Boxes/Carton
Trademark: Wegosuture or OEM brand
Transport Package: Aluminum Pack and Blister Pouch with Sterilize Ind
Specification: Ep, Usp
Origin: China
HS Code: 9018322000
CE and FDA approved Absorbable sutures by full US and UK material. WEGOSUTURES conformity with the USP and EP standard. WEGOSUTURE is soft and easy for safe knot, absorbed by the tissues with hydrolysis after implant, safe and without histological reactions. Sterile by EO conformity with ISO11137 by a CE marked sterilize center. Product will finish verification of five years validity
WEGO-PGA Features
Structure Multifilament, braided
Chemical composition 100%polyglycolic acid
Coating Polycaprolactone + Calcium Stearate
Color Violet or Undyed
Sizes USP 2-USP 8/0 metric 5 - metric 0.4
Knot tensile strength Retention 14 days post implantation 60-70%
18 days post implantation 50%
21 days post implantation 40%
Mass absorption Degradation by hydrolysis within 60-90 days
Indications General surgery, Gastroenterology, Urology, Gynaecology, Ophthalmic, Plastic and Paedriatic surgery
Sterilization Ethylene oxide


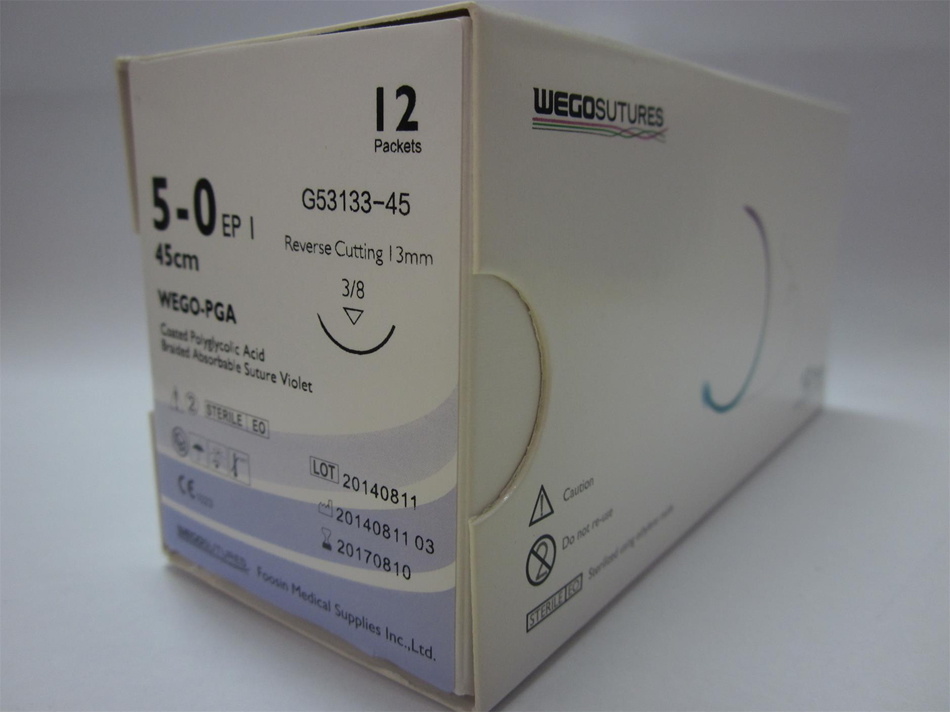 CE and FDA approved Absorbable sutures by full US and UK material. WEGOSUTURES conformity with the USP and EP standard. WEGOSUTURE is soft and easy for safe knot, absorbed by the tissues with hydrolysis after implant, safe and without histological reactions. Sterile by EO conformity with ISO11137 by a CE marked sterilize center.
CE and FDA approved Absorbable sutures by full US and UK material. WEGOSUTURES conformity with the USP and EP standard. WEGOSUTURE is soft and easy for safe knot, absorbed by the tissues with hydrolysis after implant, safe and without histological reactions. Sterile by EO conformity with ISO11137 by a CE marked sterilize center. Product will finish verification of five years validity
WEGO-PGA Features
Structure Multifilament, braided
Chemical composition 100%polyglycolic acid
Coating Polycaprolactone + Calcium Stearate
Color Violet or Undyed
Sizes USP 2-USP 8/0 metric 5 - metric 0.4
Knot tensile strength Retention 14 days post implantation 60-70%
18 days post implantation 50%
21 days post implantation 40%
Mass absorption Degradation by hydrolysis within 60-90 days
Indications General surgery, Gastroenterology, Urology, Gynaecology, Ophthalmic, Plastic and Paedriatic surgery
Sterilization Ethylene oxide



Non-Pvc Iv Bag,Infusion Bag,Iv Infusion Bag
Glass Ampoule,Caps Or Seals,Moulded Vials Co., Ltd. , http://www.ns-pharmaceutical.com